Which of the Following Is Not a Valence Bond Concept
They are found in the atoms outermost shell where the force of attraction from the nucleus is the weakest. To form the Lewis structure of Sulfur Dioxide we need first to determine the number of valence electrons available.

10 6 10 7 Valence Bond Theory Orbital Overlap Hybridization Of Atomic Orbitals Youtube
These valence electrons act as the building blocks of the structure.
. Using Formal Charge to Predict Molecular Structure. SO2 Valence Electrons. The arrangement of atoms in a molecule or ion is called its molecular structureIn many cases following the steps for writing Lewis structures may lead to more than one possible molecular structuredifferent multiple bond and lone-pair electron placements or different arrangements of atoms for instance.
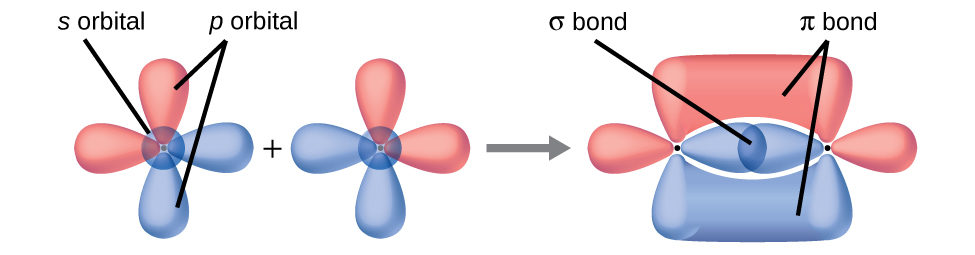
1 6 Valence Bond Theory And Hybridization Organic Chemistry I

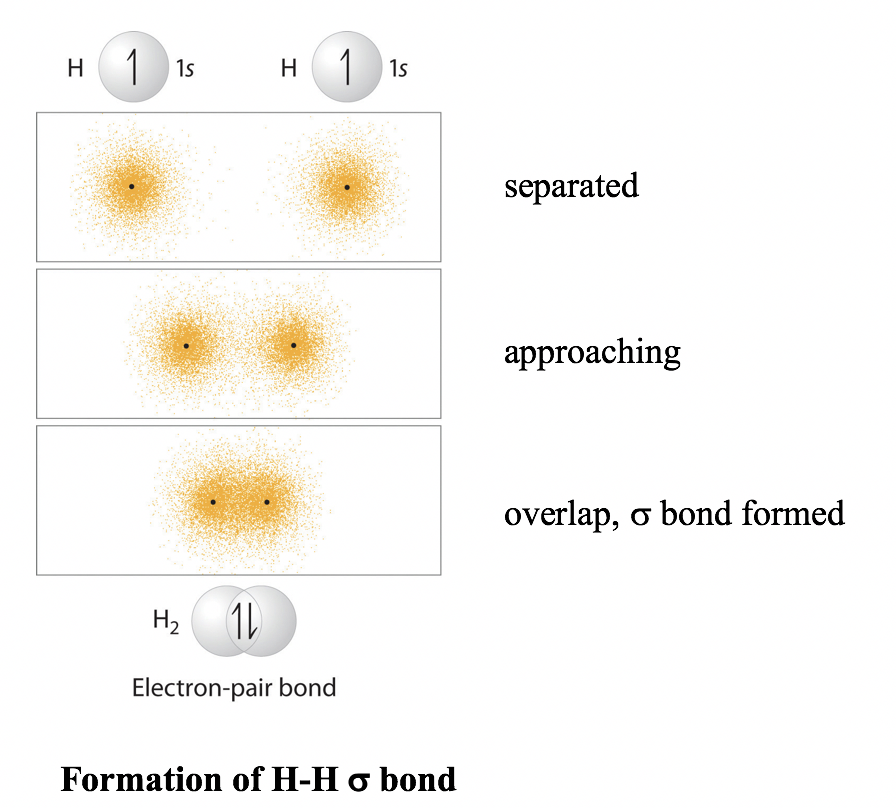
8 1 Valence Bond Theory Chemistry
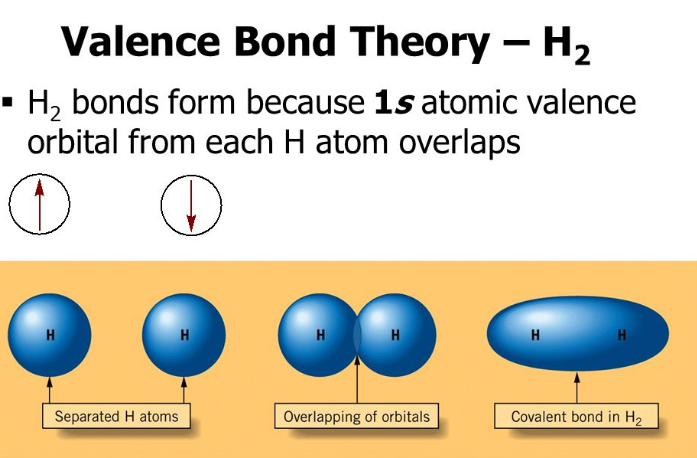
10 Difference Between Valence Bond Theory Vbt And Molecular Orbital Theory Mot With Examples Viva Differences
No comments for "Which of the Following Is Not a Valence Bond Concept"
Post a Comment